Electrocatalytic Oxygen Evolution Reaction for Energy Conversion and Storage a Comprehensive Review
Abstract
Computational calculations and experimental studies reveal that the CoOOH phase and the intermediate-spin (IS) land are the key factors for realizing efficient Co-based electrocatalysts for the oxygen evolution reaction (OER). Nevertheless, according to thermodynamics, general cobalt oxide converts to the CoOtwo stage under OER condition, retarding the OER kinetics. Herein, nosotros demonstrate a elementary and scalable strategy to fabricate electrodes with maintaining Fe-CoOOH phase and an IS state nether the OER. The changes of phase and spin states were uncovered past combining in-situ/operando X-ray based absorption spectroscopy and Raman spectroscopy. Electrochemical reconstruction of chalcogenide treated Co foam affords a highly enlarged active surface that conferred first-class catalytic action and stability in a large-scale water electrolyzer. Our findings are meaningful in that the calculated results were experimentally verified through the operando analyses. Information technology also proposes a new strategy for electrode fabrication and confirms the importance of real active phases and spin states under a particular reaction condition.
Introduction
Water electrolysis is a clean and useful technology for producing hydrogen, which is a renewable culling to fossil energiesone,2. However, the oxygen evolution reaction (OER) is a bottleneck in realizing efficient h2o electrolysis owing to its sluggish kinetics3,4,5,vi. The use of noble metal-based catalysts such as Ir and Ru oxides can accelerate the OER kineticsvii,8. However, the loftier cost and scarcity of noble metals limit their industrial application in water electrolysis. Thus, significant computational and experimental studies accept been conducted to develop efficient and cost-effective OER catalysts with globe-abundant metals.
In this regard, Co-based catalysts accept attractive meaning attention for awarding in alkaline metal OER owing to their high OER action, low price, and the mixed oxidation country, which enables the generation of various Co-based materials. Various cobalt compounds such as CoO, Co3Oiv, Co4Ofour, cubane, cobalt hydroxide, cobalt oxyhydroxide, perovskite, cobalt phosphide, cobalt borate, and cobalt sulfide have been reported to exist promising OER catalysts9,ten,eleven,12,xiii,14,15,xvi,17,18,19,twenty,21,22,23. Many studies accept focused on analyzing the properties of the pristine catalysts and have conducted density functional theory (DFT) calculations using the initial state of the catalysts. Notwithstanding, the initial stage of the Co-based catalyst may transform under alkaline OER weather. For example, cobalt hydroxide is thermodynamically oxidized nether alkaline OER conditions as follows24,25:
$$3{{{\rm{Co}}}}{\left({{{\rm{OH}}}}\right)}_{2}+\,2{{{{\rm{OH}}}}}^{-}\leftrightarrow \,{{{{\rm{Co}}}}}_{three}{{{{\rm{O}}}}}_{4}+\,4{{{{\rm{H}}}}}_{2}{{{\rm{O}}}}+2{{{{\rm{e}}}}}^{-}\,(0.81\,{{{{\rm{5}}}}}_{{{{\rm{RHE}}}}})$$
$${{{{\rm{Co}}}}}_{3}{{{{\rm{O}}}}}_{4}+\,{{{{\rm{OH}}}}}^{-}+\,{{{{\rm{H}}}}}_{two}{{{\rm{O}}}}\leftrightarrow \,three{{{\rm{CoOOH}}}}+{{{{\rm{due east}}}}}^{-}\,(1.22\,{{{{\rm{Five}}}}}_{{{{\rm{RHE}}}}})$$
$${{{\rm{CoOOH}}}}+\,{{{{\rm{OH}}}}}^{-}\leftrightarrow {{{\rm{Co}}}}{{{{\rm{O}}}}}_{2}+\,{{{{\rm{H}}}}}_{2}{{{\rm{O}}}}+{{{{\rm{e}}}}}^{-}\,(1.56\,{{{{\rm{V}}}}}_{{{{\rm{RHE}}}}})$$
The transformation of the cobalt catalyst indicates that the OER activity of the catalyst is strongly dependent on the converted final phase of cobalt during the OER rather than its initial phase. Therefore, information technology is significantly important to identify the real phase of the Co-based catalysts during the OER, and this tin be accomplished using various in-situ/operando spectroscopic techniques. The transformation of the Co stage from CoO2 to CoOOH and from CoOOH to CoOx (predicted to exist Co(VI), CoOtwo) under OER conditions has already been reported using in-situ/operando Raman spectroscopy, suggesting that the real phase of Co-based catalysts for OER is CoOtwo 26,27,28. This ascertainment was consistent with the predictions based on electrochemical thermodynamics. Yet, using time-resolved Fourier transform-infra-blood-red (FT-IR) spectroscopy, Frei et al. found that the OER kinetics are delayed more than in the presence of Co(4)=O species than in the presence of Co(III)OH species29. Strasser grouping also constitute that Co3+ species in CoOx(OH)y play an important part for loftier OER activity by acting as a fast active site30,31. Co-ordinate to the recently reported Co-based catalysts with excellent OER catalytic activity, the initial different types of catalysts are converted to similar CoOOH phases under OER conditions32. Therefore, it is important to place the agile sites during actual OER and design the electrode with Co(Iii)OH species with high catalytic activity without converting to Co(Iv)=O species. Furthermore, contempo studies have revealed that not only the stage of cobalt but also its spin state affects the OER activity of Co-based catalysts33,34,35,36,37. The due east chiliad occupancy determined by the spin land is strongly affects the binding of oxygen. Shao-Horn and coworkers reported that the spin states of cobalt and due east g occupancy significantly influences the binding strength of the oxygen intermediates37. According to various DFT calculations, the intermediate spin (IS, t 2g 5 e g 1) of Co3+ has ideal due east g occupancy, which is expected to confer first-class OER operation. Thus, fabricating Co electrodes with an appropriate CoOOH phase and IS state for the OER is a key to the practical implementation of alkaline water electrolysis.
Here, we proposed a elementary and scalable strategy to catechumen a cobalt foam (CF) electrode into a highly active catalyst for the OER through sulfur and fe treatment with the goal of maintaining the CoOOH phase and IS state of the Co-based electrode under OER weather. The surface of the modified CF electrode was reconstructed to accommodate the Iron-CoOOH species under alkaline metal weather. With increasing applied potential, the Fe-CoOOH species in the modified CF electrode was retained and not transformed to CoO2. This was observed using in-situ/operando Raman spectroscopy and X-ray absorption fine spectroscopy (XAFS). In addition, changes in the spin land of the Co-based electrode under OER weather condition were observed through in-situ/operando almost edge X-ray absorption fine structure (NEXAFS). The prepared CF electrode underwent conversion from the low spin (LS) state to IS under OER conditions and remained in this land. These experimental operando analyses could explain the improved OER activeness of the prepared CF electrode. The synthetic method and operando analysis presented herein are a feasible road to design and implement oxidation electrodes for big-scale electrolysis systems.
Results
Synthesis and morphology of the Fe-CoOOH electrode during OER
The Fe-CoOOH electrode for the OER was fabricated past the surface reconstruction strategy under alkaline OER conditions by treating the raw CF with atomic number 26 and sulfur (Fig. 1a). To verify the effect of South and Atomic number 26 treatment, the CF later OER (CF-O) and sulfur-treated CF after OER (CF-And so) were also prepared. For the Iron handling, the CF was subjected to dip coating past soaking the CF (Fig. 1b) in 0.25 M FeCliii solution for 30 s and and then drying in an oven at 70 °C for 1 h38,39,xl. The Fe treatment (CF-Fe) led to corrosion and cracks on the CF owing to Cl ion (Fig. 1c). The CF-Fe surface was oxidized and converted to CoFexCly (Supplementary Figs. 2–4). Next, for the sulfur handling, CF-Fe was thermally treated with S powder at 300 °C for 5 min in a furnace. During the sulfur treatment, some of the S anions reacted with cobalt and iron of CF-Fe while the other South anions replaced the Cl‒ anions of CF-Fe (Fig. 1d, Supplementary Figs. 4 and 5). Co and Iron of sulfur-treated CF-Fe (CF-FeS) were softly reduced compared to CF-Fe due to the lower electronegativity of sulfur than that of chlorine (Supplementary Fig. ii). X-ray photoelectron spectroscopy (XPS) spectrum suggested that Co and S were present at an atomic ratio of 1:1.74. Afterward the S and Iron handling, electrochemical reconstruction was performed under OER conditions to produce CF-FeSO. The S and remaining Cl‒ species were dissolved and exchanged with oxygen species (Oii‒, OH‒), thereby reconstructing the highly crude structure with abundant Fe-doped Co nanosheets (Fig. 1e, Supplementary Figs. 4 and 5). The reconstruction under OER conditions oxidized more CF-FeS surface, leading to the generation of arable Co3+ species and Ironiii+ species in CF-FeSO (Supplementary Figs. two–five). Scanning electron microscopy (SEM) images in Fig. 1e and Supplementary Fig. 6 revealed that CF-FeSO possesses abundant nanosheet structures on the highly rough surface. CF-O has large Co nanosheets on the plain surface (Supplementary Fig. 7), while the surface of CF-So is highly rough, with grown abundant Co nanosheet layers which were smaller than those in CF-O (Supplementary Fig. 8). Transmission electron microscopy (TEM) images of CF-FeSO showed the formation of thin nanosheets with an oxidized structure on the CF surface, such as layered double hydroxide structure (Fig. 1f, m). Energy dispersive 10-ray spectroscopy (EDS) of the TEM and SEM images reveals the homogenous incorporation of Fe on the CF despite the crude dip blanket process (Fig. 1h and Supplementary Fig. 9). As shown in the EDS chemical element mapping result (Supplementary Fig. 10), compared to the inside of CF-FeSO, the element ratio of Due south and Cl on the surface decreased while the ratio of O increased, confirming that South and Cl species were exchanged with O species. Thus, this easy and scalable procedure successfully converted raw CF into non only a substrate of the electrode but also into a catalytically agile site with a large surface area. Furthermore, the electrochemical reconstruction strategy could be adopted for cobalt oxide nanoparticles (CPs) instead of CF. The X-ray diffraction (XRD) patterns and TEM images of CP-FeSO bespeak well reconstructed structure, suggesting the high flexibility of this strategy (Supplementary Figs. eleven and 12).
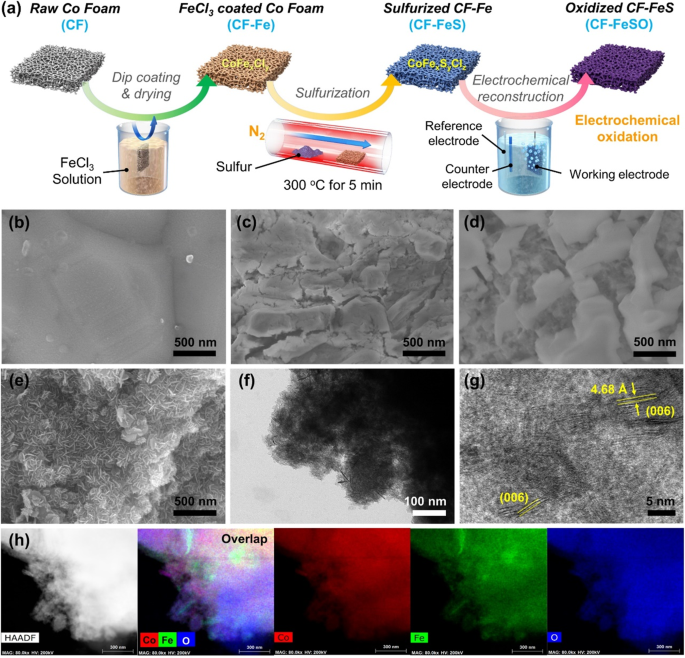
a Schematic of the fabrication of the sulfurized CoFe electrode after electrochemical oxidation (denoted as CF-FeSO). SEM images of the electrode at each step during the fabrication of the CF-FeSO electrode: b Raw CF, c CF-Fe, d CF-FeS, and due east CF-FeSO. f, thou 60 minutes-TEM images of CF-FeSO, h Free energy dispersive Ten-ray spectroscopy (EDS) elemental maps of CF-FeSO using TEM.
Characterization of fabricated CF electrode
To better sympathize the formation of CF-FeSO, surface-sensitive characterization techniques such as NEXAFS spectroscopy and Raman spectroscopy were employed (Fig. 2a, b, c). The Co 50-edge of CF-O exhibits peaks corresponding to Co2+(Td) and Co2+(Oh) with the 3d7 configuration (Fig. 2a), indicating that the CF-O surface contains CoO41. This electronic construction can also exist inferred from the O Thousand-border NEXAFS spectrum, which represents the hybridization of the Co threed and O iip orbitals42,43. The configuration of unfilled t2g orbitals and the extremely small pigsty of the e chiliad orbitals of CF-O leads small acme a t 530 eV, which could be related to the high spin (HS) state of the Co2+-t 2g 5 e chiliad two orbitals (Fig. 2b). CF-SO possess Co3+(Oh) instead of Cotwo+(Oh), suggesting that the ratio of Co3+(Oh) and Co2+(Td) is one.56:1. Co3Ofour has a spinel structure, i.e., Co2+(Td)i Coiii+(Oh)2Oiv. Thus, the state of CF-So is between CoO and CothreeOfour. The O K-edge of CF-And then shows no t2g peak and more intense eastwardg orbital peaks than that of CF-O owing to the presence of low spin Co3+(Oh) with t2g half-dozenegrand 0 orbital configuration. The Co L-edge of CF-FeSO demonstrates more than intense Co3+(Oh) peaks than that of CF-So, suggesting substantial contribution from the 3d6 configuration. The ratio of Coiii+(Oh) and Co2+(Td) was ii.32:1, indicating that CF-FeSO is a mixture of CoOOH and Co3Ofour. The only e g orbital peaks of CF-FeSO were observed in the O K-edge, suggesting completely filled t 2g orbitals. This suggests that CoOOH is present in the LS (t 2g 6 e chiliad 0 ) land in CF-FeSO. The Raman spectra farther ostend the stage of the CF-based electrode (Fig. 2c). The Raman spectrum of CF-O exhibits three peaks corresponding to E1000, F2g, and A1g, revealing the CoO phase44. The spectrum of CF-And so also shows three peaks corresponding to Eone thousand of CoOOH, Eg of Co(OH)2, and A1g of Co3O4, indicating a mixture of Co(OH)2, CothreeO4, and CoOOH. Two peaks, corresponding to Eg of CoOOH and A1g of Co3Ofour, were observed in the spectrum of CF-FeSO45.
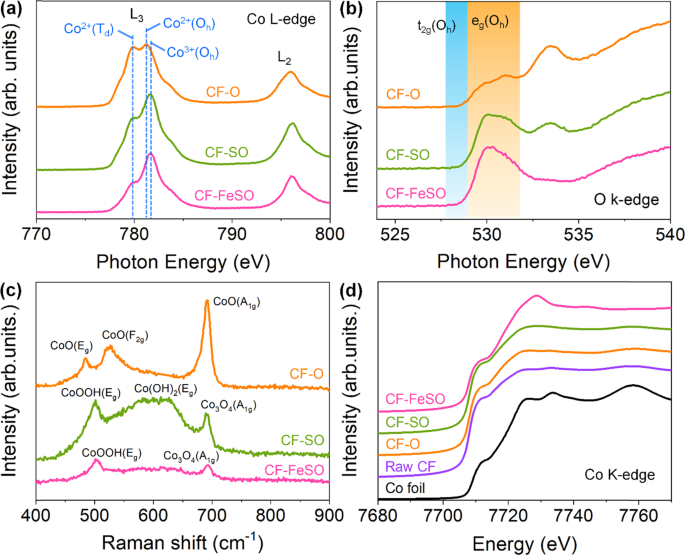
a Co L-border near edge Ten-ray absorption fine structure (NEXAFS) spectra of CF-O, CF-SO and, CF-FeSO. b O K-border NEXAFS spectra of CF-O, CF-SO and, CF-FeSO. c Raman spectra of CF-O, CF-SO and, CF-FeSO. d Co K-edge XANES of Co foil, Raw CF, CF-O, CF-SO and, CF-FeSO.
To observe the unabridged Co electronic land in the CF-based electrode, Co K-edge X-ray absorption near edge structure (XANES) spectroscopy, which is a bulk-sensitive technique, was performed (Fig. 2d). The spectrum of raw CF, which has an almost metallic character, was different from that of a Co foil because of the self-absorption due to the thick Co foam. Generally, the pre-border peak (1s → 3d for Co) is more authentic for estimating the oxidation state. However, a huge amount of Co metallic was present in the CF sample, leading to difficulty in acquiring the pre-edge and EXAFS spectra. Thus, we simply used peaks of the white line, which was related to the is to fourp transition. The oxidation state of Co was calculated from the white line peak position and the Co oxidation linear function using a standard reference (Supplementary Fig. 13). The XANES spectrum of CF-O is almost similar to that of raw CF, merely a minor broad peak is observed at 7722 eV. This suggested that a highly thin Co oxide layer was formed on the CF surface subsequently the alkaline oxidation. The spectrum of CF-SO peaked at 7725 eV and was more than intense and higher than that of CF-O. This indicates that the Co oxidation country of CF-SO is higher due to the greater number of active Co species compared to that of CF-O. This indicates that the Co oxidation state of CF-SO is college due to the greater number of active Co species compared to that of CF-O. These morphological and electronic structural label results indicate that S increases the surface roughness and converts the CF electrode to a college oxidation state nether alkaline metal OER atmospheric condition. In general, metallic Co is converted to CoO under alkaline OER atmospheric condition, which is consistent with the results obtained for CF-O. Sulfur treatment forms electrodes with a loftier proportion of cobalt sulfides, including S element, which are less electronegative compared to oxygen. Under alkaline OER conditions, Southward is dissolved and exchanged with oxygen species (O2‒, OH‒), reconstructing the oxygen-arable construction with high Co oxidation country. Cl ions nowadays in Iron treatment also human activity like S ions, forming abundant active Co3+ species on the rough CF surface.
Electrochemical backdrop and water splitting cell test of the fabricated CF electrode
We examined the OER catalytic activeness of the CF-based electrodes in O2-saturated 1 Yard KOH electrolyte using a conventional iii-electrode organisation. The cyclic voltammetry (CV) curves in Fig. 3a reveal that CF-FeSO exhibited much higher OER activity than CF-And then and CF-O. The overpotential of each electrode at 10 and 100 mA cm–2 is illustrated in Fig. 3b. CF-FeSO exhibits depression overpotentials of 192 and 230 mV at current densities of 10 and 100 mA cm–2, respectively, which are considerably lower than those of CF-SO (266 and 323 mV) and CF-O (354 and 419 mV). Moreover, the Tafel slope for CF-FeSO was xl.1 mV dec–1, which is less than those of CF-SO (56.1 mv dec–1) and CF-O (65.v mV dec–1) (Fig. 3c). The overpotentials and Tafel slopes indicated that Atomic number 26 and S treatment of the CF enhanced its intrinsic catalytic action for the OER. To investigate the reasons for the enhanced OER action of CF-FeSO, we calculated the internal and external voltammetric accuse densities and the electrochemical porosities past CV at different scan rates (Fig. 3c, d, Supplementary Figs. 14 and xv)46,47,48. Internal and external voltammetric charge densities illustrate theoretical charge of inside and surface, respectively. Electrochemical porosity is divers as the ratio of internal voltammetric charge to external voltammetric accuse, showing roughness of electrode. The detailed calculation methods are shown in SI. The results of calculated charge density and electrochemical porosity suggested that S treatment not only significantly increased the electrode roughness, but also produced arable catalytically active sites. These results are consistent with the CV expanse and XANES spectra. In the S handling footstep, the Due south anion is incorporated into the interior of CF then replaced by oxygen species equally the sulfur dissolves in the alkaline OER conditions. This phenomenon significantly increases electrochemical porosity of CF-S. In addition, in the Atomic number 26 treatment stride, Cl anion play a similar function to Southward anion to form a highly porous surface and expand the active expanse of CF-FeSO. To separately elucidate the effect of surface area and intrinsic catalytic activeness, we compared the OER activities of nanopowdered CP-O and CP-FeSO and their results are shown in Supplementary Fig. 16. In the instance of the nanopowder-blazon, there is no significant change in the physical structure. The electrochemically active surface areas (ECSA) of CP-FeSO are slightly higher than that of CP-O, every bit shown in Supplementary Fig. 17. Despite a little deviation of ECSA, CP-FeSO showed significantly improved OER activity than CP-O, suggesting that the reconstituted active sites of CP-FeSO had enhanced intrinsic catalytic activity for OER. Therefore, the cream type CF-FeSO electrode showed improved OER activity due to the synergistic effect of first-class intrinsic OER activity and large surface surface area.
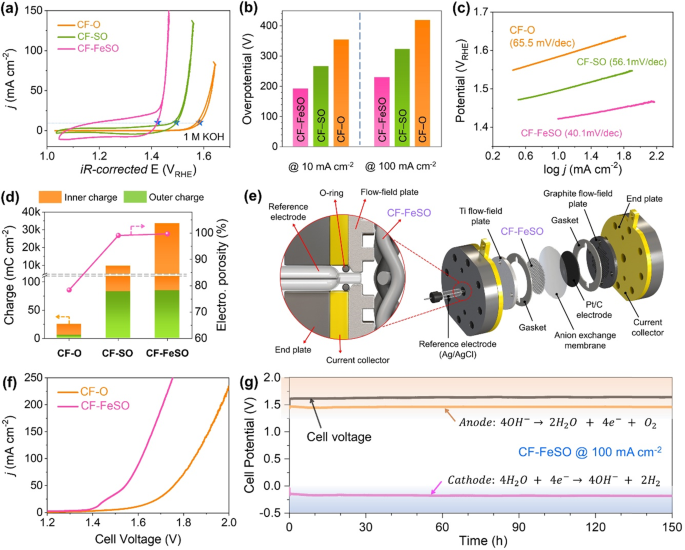
a Electrocatalytic OER activeness of CF-based electrode in 1 Grand KOH. b OER activities of the catalysts expressed as overpotentials required for 10 and 100 mA cm–2. c Tafel slopes for CF-based electrode. d Internal and external voltammetric charge densities of the CF-based electrode. e Scheme of water electrolysis single cell. f LSV of CF-O and CF-FeSO in water electrolysis unmarried jail cell. g Durability test in water electrolysis single cell. Cell voltage, anode potential, and cathode potential at 100 mA cm–2 in 1 Yard KOH.
We roughly controlled the Fe content of CF-FeSO by adjusting the concentration of FeCl3 solution (Supplementary Fig. eighteen). Then the OER performance co-ordinate to the Iron content of the prepared electrodes was evaluated and shown in Supplementary Fig. 19. Interestingly, the CV area increased with increasing FeCl3 concentration because the Cl ions reconstituted the CF surface and, thus, the surface area increased. These results lead to a not-linear human relationship between the Fe content of CF-FeSO and the FeClthree concentration. The OER activity was similarly increased when the FeCl3 concentration was between 0.05 ~ 0.five M, which means that the incorporated Fe enhances the intrinsic activity for OER, but Atomic number 26 content to a higher place a sure amount no longer effects OER operation. At a FeCl3 concentration above 1 K, OER performance deteriorates due to corrosion of CF substrates.
To ensure stability, scalability, and feasibility at an industrial scale, single water electrolysis jail cell test was conducted in 1 Thousand KOH with Pt/C electrode as a cathode electrode. Details of the water electrolysis prison cell with reference electrode for detecting anode and cathode potentials are described in Fig. 3f. CF-FeSO exhibited a prison cell voltage of 1.61 5 at a current density of 100 mA cm‒ii, which is 230 mV lower than that of CF-O (Fig. 3g). Figure 3h shows the jail cell voltage, anode potential, and cathode potential of the alkaline metal water electrolysis cell with the CF-FeSO electrode during the 150 h measurement at 100 mA cm–two. The cell voltage of CF-FeSO was maintained at 1.62 V with a depression overpotential of 200 mV for the OER, indicating remarkable stability of CF-FeSO. As shown in Supplementary Fig. 20, the SEM and EDS results confirmed that the structure and limerick of CF-FeSO were also well maintained after the durability tests. This confirmed the stability and activity of the CF-FeSO electrode in the big-scale h2o electrolysis.
In-situ/operando study for observing stage and spin state of the fabricated CF electrode
To identify the bodily OER active site of the CF-based electrode, nosotros employed in-situ/operando techniques such equally Raman, XANES, and NEXAFS spectroscopies using a customized electrochemical cell (Fig. 4a, b). The phase of cobalt materials can be transformed by varying the potential and pH, suggesting that the electronic construction of cobalt materials under OER atmospheric condition volition change with respect to that at the initial state. Thus, in-situ/operando techniques are necessary for identifying the bodily agile site of the Co-based goad. For in-situ/operando Raman spectral measurements, the laser has to be focused on the sparse electrolyte-coated CF-based electrode to identify the stage of the electrode surface during the OER. The Raman spectrum of CF-O (Fig. 4c) exhibits the CoO phase. The CoO (Ethousand and F2g) peaks are transformed into the CoOOH (Eastwardg) and Co3Ofour (A1g) peaks when CF-O is dipped in i Thou KOH solution under OCV condition. When the potential is increased to 1.53 Five, intensities of the CoOOH (Due eastg) and Co3O4 (A1g) peaks decrease and that of the CoOten (predicted to exist CoOii) superlative is increases28. This trend was likewise observed in repeated tests, demonstrating the reversibility of transformation (Supplementary Fig. 21). The transformation CF-O (Co(II)O → Co(3)OOH + Co(II, III)iiiO4 → Co(Half dozen)O2) due to increased potential in the alkaline solution is largely consistent with the thermodynamic theory discussed in the introduction. These results bespeak that CoOii is the actual active site of CF-O for the OER. On the contrary, the CoO2 species could not exist detected in the operando Raman spectrum of CF-FeSO (Fig. 4b). Nether OCV conditions, the Co3Ofour (A1g) meridian disappeared, and only the CoOOH (Eastone thousand) summit was detected. It suggests that CoOOH is a stable phase of CF-FeSO in the alkaline metal solution. Even if the practical potential for the case of CF-FeSO increases to i.53 V, only the CoOOH peak is detected, demonstrating that Atomic number 26-CoOOH is the master active site for the OER. Several Fe-CoOOH and CoiiiO4 species formed upon Iron and S treatment were hands converted into the single Fe-doped CoOOH phase, which was retained, and which deed as an OER active site nether the OER weather condition.
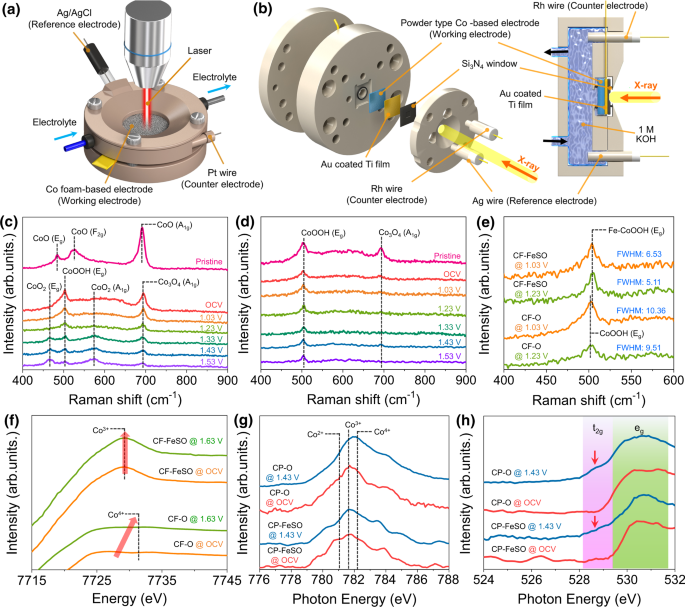
Schematic illustration of the in-situ/operando (a) Raman spectroscopy setting and b NEXAFS setup. c In-situ/operando Raman spectrum of the CF-O electrode. d In-situ/operando Raman spectrum of the CF-FeSO electrode. e In-situ/operando Raman spectrum of the CF-based electrode for comparison the CoOOH top. f In-situ/operando Co K-edge XANES spectra of the CF-O and CF-FeSO electrodes. grand In-situ/operando Co 50-edge NEXAFS spectra of the CP-O and CP-FeSO catalysts. h In-situ/operando O Chiliad-border NEXAFS spectra of the CP-O and CP-FeSO goad.
To understand the effect of S, Cl, and Fe element in reconstruction procedure, CF-ClSO was manufactured by using HCl instead of FeClthree and in-situ/operando Raman spectrum of CF-So and CF-ClSO was acquired (Supplementary Figs. 22 and 23). In the absence of a CoO10 superlative, the main phase of CF-And so was determined to be CoOOH and Co3O4. S treatment results in a high oxidation country of cobalt and an oxygen-abundant structure for the reconstructed CF electrode. These backdrop confer enhanced structural stability and inhibit the conversion to CoO2 species. The single CoOOH phase of CF-ClSO suggests that the function of Cl is promoting the conversion to the CoOOH phase by accelerating reconstruction and the role of Iron is only modulating electronic structure of Co for OER. The OER performance of CF-ClSO clearly reveals furnishings of Cl and Fe chemical element (Supplementary Fig. 23). To verify the peaks in the Raman spectrum, isotopic substitution using D2O instead of H2O was conducted. The observed DtwoO peak confirmed the in-situ status and isotropic substitution experiment (Supplementary Fig. 24). The CoOOH (Eg) tiptop was bluish-shifted, suggesting the replacement of hydrogen with deuterium (Supplementary Figs. 25 and 26). In contrast, the CoO10 peaks showed no shift in the isotopic substitution experiment, suggesting that there was no contribution from hydrogen in the CoOii acme, which is consistent with a previous report29. The CoOOH peaks of CF-FeSO were red-shifted with respect to those of CF-O because of the larger radius of Fe3+ than Coiii+ (Fig. 4e). This confirmed the Fe-CoOOH phase of CF-FeSO under the OER conditions. The OER intermediates were as well observed by in-situ/operando Raman spectroscopy (Supplementary Fig. 27). Peaks corresponding to the superoxo species (OO–) were observed for the CF-O electrode; however, CF-FeSO showed no peaks for the OER intermediates. This suggests that the rate determining step (RDS) in the OER of CoO2 was the release of O2 28 whereas, RDS of Atomic number 26-CoOOH would exist the deprotonation step. The oxidation state of the Co-based electrode measured by in-situ/operando XAFS can farther confirm the transition of the Co stage under OER atmospheric condition. As shown in Fig. 4f and Supplementary Figs. 28–xxx, the change in the XANES spectrum is small due to the metallic Co substrates. The minor and broad XANES summit of CF-O shifted to 7725 eV at OCV, suggesting that the surface was oxidized in alkaline solution. When the practical potential increases to one.63 V, the peak shifts to a higher energy, indicating a phase transition of Co at the surface. In contrast, CF-FeSO showed stable oxidation states of Co3+ and Ironthree+ from OCV upwardly to 1.63 V (Supplementary Fig. 31), confirming that the Atomic number 26-doped CoOOH sites maintained stable phases under harsh OER status. These trends are also observed in nanopowder-blazon samples (Supplementary Fig. 32), and then the results of XANES back up the observations of in-situ/operando Raman spectroscopy.
To closely observe the spin state and oxidation states at goad surface nether OER conditions, we conducted in-situ/operando NEXAFS spectroscopy. The foam blazon electrode is considerably difficult to accommodate in the in-situ/operando NEXAFS cell owing to the harsh surface. We prepared CP-FeSO pulverisation using a method similar to the synthesis method of CF-FeSO and compared it with CP-O. The catalyst was loaded on twenty nm Au, and ten nm Ti was coated on 100 nm SiN window. The goad-coated SiN window was attached to a customized NEXAFS cell (Fig. 4b). Under OCV conditions, CP-O exhibited Coii+ and Coiii+ peaks with filled t 2g orbitals, indicating that CP-O independent Cotwo+ and Co3+ (LS) species (Fig. 4g, h). When the potential increased to i.43 V, the Co L-edge top of CP-O was shifted to higher energy, with a clear appearance of the t 2g pinnacle (O Chiliad-edge spectrum) of CoO. The shifted wide Co L-edge peak indicates a mixture of Cotwo+, Co3+, and some converted Co4+(CoO2) species. These results agreed with the in-situ/operando Raman spectrum. The t 2g peak of CP-O suggests that the CoO2 generated during the OER would have a HS (t 2g three e g 2) state. CP-FeSO besides exhibited a higher spin country under OER conditions, but the trend was slightly different. At i.43 V, the Co Fifty-edge of CP-FeSO most maintained the position of the main peaks with decreasing Co2+ species, indicating a high proportion of the Cothree+ state (Fe-CoOOH) of the CP-FeSO catalyst under OER weather condition. Despite the high proportion of Coiii+ species, a small t2g superlative of CP-FeSO was detected. This suggested that Fe-CoOOH in CP-FeSO transformed to an intermediate spin (IS, t 2g 5 e g 1) state from the LS state during the OER. All the cobalt catalysts were converted to the loftier spin state under OER conditions. The increased intensity of the t 2g peak of the O G-border NEXAFS was also observed in in-situ/operando NEXAFS study of an iridium catalyst under acidic conditions49. Pfeifer et al. reported that baggy iridium oxide possesses electrophilic oxygen under OER conditions. We anticipated that a similar miracle occurs in the Co-based catalyst nether element of group i OER conditions. The oxygen species of the Co-based catalyst are expected to have a higher electrophilic character during the OER and are converted to weaker field ligands, thus resulting in the college spin country of the cobalt goad under OER conditions.
Origin of the high OER performance of CF-FeSO
The OER performance of the CF-based electrode is highly affected past he backdrop of the active sites under OER weather condition. The estimated real active sites and their estimated electronic configurations during the OER are shown in Fig. v. The CoO2 stage in CF-O, for which the active site is Co4+ nether OER, exhibited 3d5 valence electronic configuration with a HS state. Shao-Horn et al. proposed that the optimal e chiliad -orbital occupancy for the bounden of OER intermediates is almost 1.237. The HS CoO2 phase has a one-half-filled e g orbital, with an occupancy of ≈ 2. This is higher than the optimal value and renders the electrophilic oxygen lattice equally a weaker field ligand. Furthermore, the CoOii stage with half-filled e chiliad orbital has weak bonding with the adsorbed oxygen intermediates, thereby enhancing their electrophilicity50. These electronic structures of the HS CoO2 could stabilize the electrophilic superoxo intermediates (OO–) to a college extent, as observed by Raman spectroscopy (Supplementary Fig. 27). Owing to this, the ho-hum dioxygen release becomes the RDS, thus explaining the low OER activeness of CF-O. On the other mitt, the CF-FeSO electrode possessed arable Iron-CoOOH species under real OER conditions without changing to the CoO2 species. Magnussen et al. analyzed the stable CoOOH construction by operando surface X-ray diffraction25. Defects and border sites of the Co materials are expected to initiate the phase transition. The CoOOH (100) flick possesses the ideal 3-fold coordinated µ3-O site with very low defects (µtwo-O site), resulting in a stable phase in the OER reaction. In the in operando Raman spectrum, the CoOOH pinnacle of CF-FeSO was sharper than that of CF-O, suggesting that the reconstructed Fe-CoOOH in alkaline media has lower defects than the phase-transferred CoOOH (Fig. 4d)21. This effect means that under OER weather, a stable Fe-CoOOH phase of CF-FeSO is produced, which is equivalent to the elaborately prepared CoOOH25. Furthermore, the Fe-CoOOH phase of CF-FeSO inverse to an IS state (t 2g 5 e g 1) from the LS state (t 2g 6 eastward g 0) under OER conditions, every bit observed by in-situ/operando NEXAFS spectroscopy. Recent studies advise that the Fe-substituted IS land of Co3+ leads to optimal filling of the eastg orbital and enhanced covalency, thus promoting the OER catalytic activity33,34. The IS state of CoOOH (Co3+) showed ane of eastg-orbital occupancy, possess slightly strong adsorption energy for the OER. The HS state of Fe3+ exhibited the 3dv (t 2g 3 due east 1000 2) configuration, which is expected to accept more filled eastone thousand orbitals and weaker bonding with the adsorbed oxygen intermediates than IS state of CoOOH. We expected that the local configuration of Fe3+ and CoOOH could lead to a modulated electronic structure with optimal adsorption energy of OER intermediates, leading to loftier OER catalytic action51. The RDS of Fe-CoOOH is deprotonation pace which suggests thermodynamically favorable chemical step for OER52, revealing this optimal electronic construction. Therefore, the CF-FeSO electrode possesses many stable Iron-CoOOH phases with IS states during the OER attributable to reconstruction in alkaline solution; These phases are expected to have the proper electronic structure to provide optimal adsorption energy to the OER intermediates, resulting in loftier OER performance. These results testify that the phase and spin state of the existent active sites significantly influence the catalytic activity. Furthermore, no elaborate engineering of the pristine catalyst is required. This facile electrochemical reconstruction for fabricating electrode is an effective strategy at an industrial scale for improving the OER functioning.
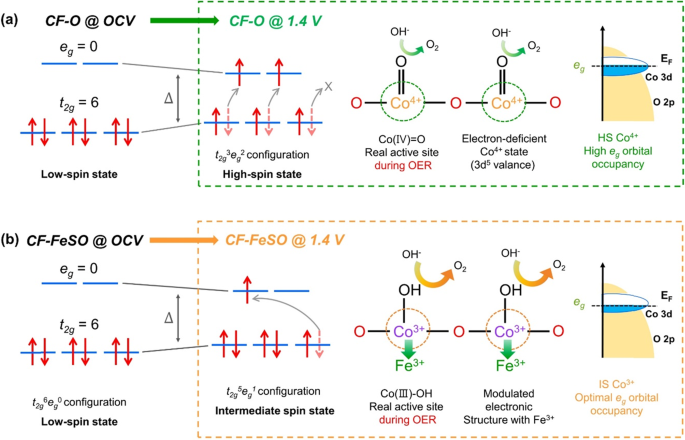
Spin country under OCV and OER weather condition and chemic phase and Co 3d– O 2p overlap under OER conditions for a CF-O and b CF-FeSO. Transparent dotted arrows point the state before the OER and red arrows represent the converted spin state during the OER.
Discussion
In this work, nosotros demonstrate a facile and efficient strategy to fabricate a highly active and scalable electrode for the OER, without requiring detailed engineering of the catalyst. The CF-FeSO electrode was manufactured past fe dipping, sulfur heat treatment, and reconstruction under OER conditions. CF-FeSO exhibited remarkable OER action (192 mV at ten mA cm‒two and 230 mV at 100 mA cm‒ii) and durability (150 h at 100 mA cm‒ii) not only for a three-electrode arrangement but also for a large-scale water electrolysis cell. CF-FeSO has a highly porous and crude surface, leading to an enlarged active surface area. Nether alkaline metal conditions, the active site of CF-FeSO is reconstructed to the Fe-CoOOH species. In-situ/operando Raman spectroscopy and XAFS spectroscopy prove that the Fe-CoOOH species, known to be an intrinsically highly active species for the OER, was retained nether the OER conditions and did not catechumen to the CoO2 species, which take depression activeness. Based on the results of in-situ/operando NEXAFS spectroscopy, the spin state of the Co-based catalyst increased nether the OER operating weather. Especially, Fe-CoOOH was converted to the IS state from LS state, resulting in optimal electronic construction and free energy of the OER intermediates. This Fe-CoOOH phase and IS spin state of CF-FeSO under the reaction conditions lead to superior OER functioning. Our piece of work emphasizes the importance of real active sites during the OER and provides a new viewpoint for the fabrication of electrode by means of electrochemical reconstruction.
Methods
Materials
KOH (90% flake), sulfur, deuterium oxide (D2O), and FeCliii were purchased from Sigma Aldrich. CF was bought from alantum. The DI water used in this work was prepared using an arium mini lab water system (Sartorius). The anion exchange membrane (AEM, Sustainion® X37-50 grade T) for the unmarried jail cell exam was purchased from Dioxide Materials. All products were used as received without further purification.
Grooming of Co foam-based electrodes
A piece of CF (1 × 2 cm2) was done with DI water and dried nether Due north2. For the iron treatment, 0.25 Yard FeCl3 solution was deposited on the porous CF by a dip blanket process and so dried using a convection oven at 70 °C (denoted as CF-Fe). The prepared CF-Fe electrode was sulfurized by rut treatment with sulfur. Sulfur pulverization (200 mg) and Fe-CF were put into each side of a quartz gunkhole. The quartz boat with sulfur powder and Fe-CF was put into the middle of a furnace tube. The side of the quartz boat with CF was placed downstream of the furnace. The furnace temperature was raised to 300 °C (at x °C/min) and maintained for 5 min. The furnace was cooled down to room temperature. The electrochemical reconstruction process was conducted in a three-electrode arrangement with iron and sulfur-treated CF (denoted equally CF-FeS) every bit the working electrode, graphite electrode every bit the counter electrode, and Hg/HgO as the reference electrode. For electrochemical reconstruction procedure, a current density of 100 mA cm‒2 was practical for x min. To check the completion of electrochemical reconstruction, circadian voltammetry was performed in the potential range 1–1.8 V vs RHE at 100 mV s‒ane for 50 cycles, 10 mV due south‒one for x cycles, and two mV s‒1 for 2 cycles. If the electrochemical reconstruction process is not completed, the CV will appear slanted. Afterwards the electrochemical reconstruction, the fe and sulfur-treated CF (CF-FeSO) was washed with DI h2o. The preparation process of CF-Then was same as that of CF-FeSO, except that there was no iron handling process. The grooming procedure of CF-O was same as that of CF-FeSO, except that at that place were no iron and sulfur treatment processes. The preparation process of CF-ClSO was same as that of CF-FeSO, except that 0.75 Chiliad HCl was used instead of 0.25 M FeCl3. The grooming process of pulverisation-type CP-FeSO was same every bit that of CF-FeSO, except for the starting material—commercial CoO pulverization (Sigma Aldrich) was used as the starting fabric.
Electrochemical measurements
The electrochemical tests were performed on potentiostat VSP (biologic) using a Hg/HgO electrode equally the reference electrode and graphite electrode as the counter electrode. An O2-saturated 1 Chiliad KOH solution was used every bit the electrolyte. The measured potential was calibrated to the RHE. The Hg/HgO reference electrode was calibrated using CV under hydrogen-saturated conditions. After electrochemical reconstruction, the electrolyte was replaced with a new electrolyte owing to sulfur oxidation. The catalytic activity of the fabricated electrode for the OER was measured from the CV curves at a scan rate of 2 mV south‒ane. Ohmic resistance was measured using electrochemical impedance spectroscopy from k to 0.1 Hz to compensate the iR loss. The alkali metal water electrolyzer exam was conducted using a 10 cm2 unmarried prison cell with two symmetric electrodes equally both cathode and anode. To prepare the cathode, commercial Pt/C (Tanaka Kikinzoku Kogyo 46%) was sprayed on carbon paper (Sigracet SGL 39BB). The Pt loading was 0.iv mg cm‒2. The sustainion anion substitution membrane (PTFE Supported Sustainion® 37-50, Dioxide Materials) was used to dissever both the electrodes. KOH solution (1 M) was allowed to flow to the anode and cathode sides using a peristaltic pump. The Ag/AgCl (3 Thousand NaCl) reference was adjusted to the anode-side for measuring the cathode and anode potentials. The cathode and anode potentials were calculated using the following equation: Jail cell voltage = anode potential – cathode potential – IR, where I and R are the measured electric current and ohmic resistance, respectively.
Textile characterization
For characterization of the electrode, Raman spectroscopy (Renishaw) was conducted at room temperature using a 785 nm light amplification by stimulated emission of radiation for identifying the phase of the cobalt-based electrode. X-ray photoelectron spectroscopy (XPS, ULVAC PHI, VersaProbe PHI 5000) was performed to investigate the chemic land of the cobalt-based electrode. X-ray absorption spectroscopy (XAS) and near border XAS (NEXAFS) spectroscopy were conducted for analyzing the chemical state of the catalyst at the 1D and 10D beamline of the Pohang Accelerator Laboratory (PAL), Pohang, South korea. Morphology and energy dispersive spectroscopy (EDS) of the Co foam-based electrode were analyzed by SEM (Hitachi, Regulus 8230). The morphologies of the fabricated electrode and catalysts were measured by high-resolution manual electron microscopy (60 minutes-TEM, FEI Talos F200X). In-situ/operando analysis techniques are described in the Supplementary Information.
Data availability
Source information are provided with this paper.
References
-
Mallouk, T. E. H2o electrolysis: Divide and conquer. Nat. Chem. v, 362–363 (2013).
-
Stojić, D. Fifty., Marčeta, Grand. P., Sovilj, S. P. & Miljanić, Š. S. Hydrogen generation from h2o electrolysis—possibilities of energy saving. J. Power Sources. 118, 315–319 (2003).
-
Suen, N.-T. et al. Electrocatalysis for the oxygen evolution reaction: recent development and futurity perspectives. Chem. Soc. Rev. 46, 337–365 (2017).
-
Tahir, M. et al. Electrocatalytic oxygen evolution reaction for energy conversion and storage: a comprehensive review. Nano Free energy. 37, 136–157 (2017).
-
Zhao, J.-W., Li, C.-F., Shi, Z.-X., Guan, J.-L. & Li, Thousand.-R. Boosting Lattice Oxygen Oxidation of Perovskite to Efficiently Catalyze Oxygen Evolution Reaction by FeOOH Decoration. Enquiry. 2020, 6961578 (2020).
-
Feng, J. Ten., Ye, S. H., Xu, H., Tong, Y. Ten. & Li, Thou. R. Design and synthesis of FeOOH/CeO2 heterolayered nanotube electrocatalysts for the oxygen development reaction. Adv. Mater. 28, 4698–4703 (2016).
-
Seitz, 50. C. et al. A highly active and stable IrOx/SrIrO3 catalyst for the oxygen evolution reaction. Scientific discipline. 353, 1011–1014 (2016).
-
Reier, T., Oezaslan, G. & Strasser, P. Electrocatalytic oxygen evolution reaction (OER) on Ru, Ir, and Pt catalysts: a comparative study of nanoparticles and bulk materials. Acs Catal. 2, 1765–1772 (2012).
-
Esswein, A. J., McMurdo, M. J., Ross, P. North., Bong, A. T. & Tilley, T. D. Size-dependent activeness of Co3O4 nanoparticle anodes for alkaline h2o electrolysis. J. Phys. Chem. C. 113, 15068–15072 (2009).
-
Nguyen, A. I. et al. Stabilization of reactive Co4O4 cubane oxygen-evolution catalysts inside porous frameworks. Proc. Natl Acad. Sci. 116, 11630–11639 (2019).
-
Zhuang, 50. et al. Ultrathin fe‐cobalt oxide nanosheets with abundant oxygen vacancies for the oxygen evolution reaction. Adv. Mater. 29, 1606793 (2017).
-
Yuan, 10. et al. Controlled stage evolution from Co nanochains to CoO nanocubes and their application every bit OER catalysts. ACS Energy Lett. 2, 1208–1213 (2017).
-
Verma, S., Lu, 10., Ma, Due south., Masel, R. I. & Kenis, P. J. The effect of electrolyte composition on the electroreduction of CO2 to CO on Ag based gas diffusion electrodes. Phys. Chem. Chem. Phys. eighteen, 7075–7084 (2016).
-
Wang, H.-Y. et al. In operando identification of geometrical-site-dependent h2o oxidation activeness of spinel Co3O4. J. Am. Chem. Soc. 138, 36–39 (2016).
-
Ye, South. H., Shi, Z. X., Feng, J. X., Tong, Y. X. & Li, M. R. Activating CoOOH porous nanosheet arrays past fractional fe substitution for efficient oxygen evolution reaction. Angew. Chem. Int. Ed. 57, 2672–2676 (2018).
-
Han, X. et al. Ultrasensitive iron‐triggered nanosized Iron–CoOOH integrated with graphene for highly efficient oxygen evolution. Adv. Free energy. Mater. vii, 1602148 (2017).
-
Ren, Due south. et al. Molecular electrocatalysts can mediate fast, selective CO2 reduction in a menstruation cell. Science. 365, 367–369 (2019).
-
Yuan, C.-Z. et al. Cobalt phosphate nanoparticles busy with nitrogen-doped carbon layers as highly active and stable electrocatalysts for the oxygen evolution reaction. J. Mater. Chem. A. iv, 8155–8160 (2016).
-
Chen, P. et al. Strong‐coupled cobalt borate nanosheets/graphene hybrid as electrocatalyst for water oxidation under both alkaline and neutral conditions. Angew. Chem. Int. Ed. 55, 2488–2492 (2016).
-
Cai, P., Huang, J., Chen, J. & Wen, Z. Oxygen‐containing amorphous cobalt sulfide porous nanocubes as high‐activity electrocatalysts for the oxygen evolution reaction in an alkaline/neutral medium. Angew. Chem. 129, 4936–4939 (2017).
-
Xu, Z. et al. Topic review: application of Raman spectroscopy characterization in micro/nano-machining. Micromachines. 9, 361 (2018).
-
Feng, J. Ten. et al. FeOOH/Co/FeOOH hybrid nanotube arrays as loftier‐performance electrocatalysts for the oxygen development reaction. Angew. Chem. Int. Ed. 55, 3694–3698 (2016).
-
Xu, H., Shi, Z. X., Tong, Y. X. & Li, Yard. R. Porous microrod arrays constructed by carbon‐confined NiCo@NiCoO2 cadre@beat nanoparticles equally efficient electrocatalysts for oxygen evolution. Adv. Mater. 30, 1705442 (2018).
-
Binninger, T. et al. Thermodynamic explanation of the universal correlation betwixt oxygen evolution activeness and corrosion of oxide catalysts. Sci. Rep. five, 12167 (2015).
-
Reikowski, F. et al. Operando surface ten-ray diffraction studies of structurally defined Co3O4 and CoOOH thin films during oxygen evolution. Acs. Catalysis. nine, 3811–3821 (2019).
-
Joya, K. S. & Sala, 10. In situ Raman and surface-enhanced Raman spectroscopy on working electrodes: spectroelectrochemical characterization of h2o oxidation electrocatalysts. Phys. Chem. Chem. Phys. 17, 21094–21103 (2015).
-
Yeo, B. S. & Bell, A. T. Enhanced action of gold-supported cobalt oxide for the electrochemical evolution of oxygen. J. Am. Chem. Soc. 133, 5587–5593 (2011).
-
Moysiadou, A., Lee, S., Hsu, C.-S., Chen, H. M. & Hu, X. Mechanism of oxygen development catalyzed by cobalt oxyhydroxide: cobalt superoxide species as a key intermediate and dioxygen release as a rate-determining pace. J. Am. Chem. Soc. 142, 11901–11914 (2020).
-
Zhang, Yard., De Respinis, M. & Frei, H. Time-resolved observations of water oxidation intermediates on a cobalt oxide nanoparticle goad. Nat. Chem. half-dozen, 362–367 (2014).
-
Bergmann, A. et al. Unified structural motifs of the catalytically active state of Co(oxyhydr)oxides during the electrochemical oxygen development reaction. Nat. Catal. ane, 711–719 (2018).
-
Bergmann, A. et al. Reversible amorphization and the catalytically active state of crystalline Co3O4 during oxygen evolution. Nat. Commun. 6, 8625 (2015).
-
Wu, T. et al. Atomic number 26-facilitated dynamic active-site generation on spinel CoAl2O4 with self-termination of surface reconstruction for water oxidation. Nat. Catal. 2, 763–772 (2019).
-
Tong, Y. et al. Spin-land regulation of perovskite cobaltite to realize enhanced oxygen evolution activity. Chem. three, 812–821 (2017).
-
Duan, Y. et al. Tailoring the Co 3d-O 2p covalency in LaCoO3 by Fe substitution to promote oxygen evolution reaction. Chem. Mater. 29, 10534–10541 (2017).
-
Vocal, J. et al. A review on fundamentals for designing oxygen evolution electrocatalysts. Chem. Soc. Rev. 49, 2196–2214 (2020).
-
Zhou, S. et al. Technology electrocatalytic activity in nanosized perovskite cobaltite through surface spin-country transition. Nat. Commun. 7, 1–vii (2016).
-
Suntivich, J., May, Thousand. J., Gasteiger, H. A., Goodenough, J. B. & Shao-Horn, Y. A perovskite oxide optimized for oxygen development catalysis from molecular orbital principles. Science. 334, 1383–1385 (2011).
-
Zhang, C., Chen, T., Zhang, H., Li, Z. & Hao, J. Hydrated‐metal‐halide‐based deep‐eutectic‐solvent‐mediated NiFe layered double hydroxide: an splendid electrocatalyst for urea electrolysis and h2o splitting. Chem.– Asian J. 14, 2995–3002 (2019).
-
Zhang, H., Du, J., Niu, D., Hu, South. & Zhang, X. Synthesis and characterization of Fe3+ and CeO2 Co-decorated NiOOH electrocatalysts supported by nickel foam for the oxygen evolution reaction. Int. J. Electrochem. Sci. 14, 6532–6545 (2019).
-
Bai, L., Lee, S. & Hu, X. J. A. C. Spectroscopic and electrokinetic evidence for a bifunctional mechanism of the oxygen development reaction. Angew. Chem. 133, 3132–3140 (2020).
-
Wang, J., Zhou, J., Hu, Y. & Regier, T. Chemical interaction and imaging of unmarried Co3O4/graphene sheets studied past scanning transmission 10-ray microscopy and Ten-ray absorption spectroscopy. Energy Environ. Sci. six, 926–934 (2013).
-
Suntivich, J. et al. Design principles for oxygen-reduction activity on perovskite oxide catalysts for fuel cells and metallic–air batteries. Nat. Chem. 3, 546–550 (2011).
-
Klie, R. et al. Directly measurement of the low-temperature spin-state transition in LaCoO3. Phys. Rev. Lett. 99, 047203 (2007).
-
Rivas‐Murias, B. & Salgueiriño, V. Thermodynamic CoO–Co3O4 crossover using Raman spectroscopy in magnetic octahedron‐shaped nanocrystals. J. Raman Spectrosc. 48, 837–841 (2017).
-
Liu, Y.-C., Koza, J. A. & Switzer, J. A. Conversion of electrodeposited Co(OH)2 to CoOOH and Co3O4, and comparison of their catalytic activeness for the oxygen evolution reaction. Electrochim. Acta. 140, 359–365 (2014).
-
Lee, Due west. H. et al. Oxygen vacancies induced NiFe-hydroxide as a scalable, efficient, and stable electrode for alkaline overall water splitting. ACS Sustain. Chem. eight, 14071–14081 (2020).
-
Teles, J. J., Faria, East. R., Franco, D. V. & Da Silva, L. One thousand. J. Inner and outer surface areas, electrochemical porosity, and morphology cistron of mixed oxide-covered mesh electrodes with a nominal limerick of MOME-Sn0.5IrxRu0.5-xO2. Int. J. Electrochem. Sci. 12, 1755–1773 (2017).
-
De Pauli, C. & Trasatti, S. Electrochemical surface label of IrO2+ SnO2 mixed oxide electrocatalysts. J. Electroanalytical Chem. 396, 161–168 (1995).
-
Pfeifer, V. et al. In situ observation of reactive oxygen species forming on oxygen-evolving iridium surfaces. Chem. Sci. eight, 2143–2149 (2017).
-
Wang, J. et al. Ultrathin LiCoO2 nanosheets: an efficient water-oxidation catalyst. ACS Appl. Mater. Interfaces. 9, 7100–7107 (2017).
-
Zhang, B. et al. Homogeneously dispersed multimetal oxygen-evolving catalysts. Science. 352, 333–337 (2016).
-
Grimaud, A. et al. Activating lattice oxygen redox reactions in metallic oxides to catalyse oxygen evolution. Nat. Chem. 9, 457–465 (2017).
Acknowledgements
This work was supported by the Korea Institute of Science and Technology (KIST) institutional program and "Carbon to X Project" (Projection No. 2020M3H7A1098229) through the National Inquiry Foundation (NRF) funded past the Ministry of Scientific discipline and ICT, Republic of Korea. This research was also supported by the National Inquiry Council of Science & Technology (NST) grant by the Korean government (MSIT) (No. CAP21011-100) and National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (NRF-2021R1A2C2093467). We besides acknowledge Advanced Analysis Center at KIST for the TEM and XPS measurements. We wish to thank 1D XRS KIST-PAL beamline and 10D XAS-KIST beamline for measuring the hard X-ray and soft X-ray absorption spectroscopy (XAS), respectively.
Author information
Affiliations
Contributions
W.H.50. designed/conducted the experiments, analyzed the information, and wrote the manuscript. Chiliad.H.H. and Y.J.K. contributed to the electrochemical jail cell tests. B.K.M. provided an idea for the electrochemical analysis. Chiliad.H.C. and H.-Southward.O. supervised the research and wrote the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ideals declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Chuan Zhao and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional data
Publisher's note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Admission This article is licensed nether a Creative Eatables Attribution iv.0 International License, which permits use, sharing, accommodation, distribution and reproduction in whatsoever medium or format, as long equally yous requite appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and bespeak if changes were made. The images or other 3rd party material in this article are included in the article's Creative Commons license, unless indicated otherwise in a credit line to the fabric. If material is not included in the article's Creative Commons license and your intended utilise is not permitted past statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a re-create of this license, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and Permissions
Virtually this article
Cite this article
Lee, Due west.H., Han, M.H., Ko, YJ. et al. Electrode reconstruction strategy for oxygen development reaction: maintaining Fe-CoOOH stage with intermediate-spin state during electrolysis. Nat Commun 13, 605 (2022). https://doi.org/ten.1038/s41467-022-28260-five
-
Received:
-
Accustomed:
-
Published:
-
DOI : https://doi.org/10.1038/s41467-022-28260-5
Comments
Past submitting a comment you agree to abide past our Terms and Community Guidelines. If you lot notice something abusive or that does non comply with our terms or guidelines please flag information technology as inappropriate.
Source: https://www.nature.com/articles/s41467-022-28260-5
0 Response to "Electrocatalytic Oxygen Evolution Reaction for Energy Conversion and Storage a Comprehensive Review"
Post a Comment